The use of NOACs in the extended management of cancer‑associated thrombosis

Just a year after meeting her first grandchild, Claire received a lung cancer diagnosis. She was forced to give up her hobby as a frequent hiker and struggled to stay active due to the burdens of her treatment; Claire later developed a deep vein thrombosis (DVT). For the treatment of her DVT, Claire was prescribed rivaroxaban because she expressed a preference for an oral anticoagulant. Six months have passed, and Claire has not experienced a recurrent DVT nor any adverse events. A decision is now needed on whether she requires extended anticoagulation to reduce the risk of recurrent venous thromboembolism (VTE) or if treatment can be safely discontinued.
What do guidelines suggest is the best course of action to protect Claire?
The risk and benefits of extended anticoagulation need to be evaluated when making cancer‑associated thrombosis therapy decisions
Patients suffering from cancer make up approximately 20% of overall VTE incidence. Effective treatment is essential for cancer‑associated thrombosis (CAT) because it is the second biggest cause of death in patients with cancer, after the disease itself.1
Although patients with cancer face the highest risk of recurrent VTE in the first few months after cancer diagnosis, they may require protection from VTE recurrence for as long as their cancer is active.2,3 A 2019 real-world study highlighted that extended treatment can offer patients better protection from recurrent VTE than short-term treatment, with over twofold higher risk of recurrent VTE in patients with CAT treated for up to 3 months compared with those treated up to 6 months and beyond.2

Risk of VTE recurrence is higher in patients with CAT receiving 3 months of treatment compared with 6 months and beyond.
CAT, cancer‑associated thrombosis; VTE, venous thromboembolism.
The risk of bleeding events should be considered in patients with CAT using anticoagulation therapy.3,4 When choosing to extend anticoagulation therapy, cancer-related and treatment‑related risk factors need to be evaluated in order to balance the benefits of extended anticoagulation and the relative increased risk of major bleeding events in these vulnerable patients.2
International guidelines endorse NOACs for extended therapy in patients with CAT
A wealth of new data on the treatment of CAT has resulted in updated guidelines, including those for extended anticoagulation. During the 10th annual virtual International Conference on Thrombosis and Hemostasis Issues in Cancer (ICTHIC), Professor Alok Khorana (Cleveland Clinic, OH) reported: ‘updated guidelines include new recommendations for oral anticoagulants for initial, short‑term and long-term anticoagulation after diagnosis of VTE.’

ESC5, ASCO6, ITAC7, NCCN8 and ASH9 guideline recommendations for extended treatment of cancer-associated thrombosis. *Such as those with metastatic disease or receiving chemotherapy. #Conditional recommendation, very low certainty in the evidence of effects. ASCO, American Society of Clinical Oncology; ASH, American Society of Hematology; CrCl, creatinine clearance; DDI, drug–drug interactions; ESC, European Society of Cardiology; GI, gastrointestinal; ITAC, International Initiative on Thrombosis and Cancer; LMWH, low molecular weight heparin; NCCN, National Comprehensive Cancer Network; NOAC, non-vitamin K antagonist oral anticoagulant; PE, pulmonary embolism; VKA, vitamin K antagonist.
Recommendations on when to extend anticoagulation therapy for patients with CAT are based on individual assessment of the risk of recurrence.7 In general, this means that patients should continue to receive anticoagulation beyond 6 months for as long as their cancer is active, or if other risk factors persist.5,6,9,10 Patients should be re-evaluated frequently to assess riskؘ–benefit ratio of continued anticoagulation.10,11
Non-vitamin K antagonist oral anticoagulants (NOACs; including rivaroxaban) are endorsed by guidelines as an alternative to low molecular weight heparin (LMWH); when choosing between these, several factors should be considered including: tumour stage and type, risk of bleeding, potential drug–drug interactions and kidney function.7,8,10 These guidelines often recommend NOACs in patients with CAT that have a low risk of bleeding events, and caution against their use in high bleeding risk cancers, such as gastrointestinal cancer.10
Patients with CAT demonstrate an increased persistence with oral rivaroxaban versus injectable anticoagulants
Historically, LMWH has been the predominant recommendation for the treatment of CAT. However, this treatment relies on daily injections, a requirement that many may find burdensome when receiving extended therapy.12 Patient adherence to treatment is important for maintaining the effectiveness of anticoagulation treatment, and patient preferences for drug administration needs to be considered.12 Of all NOACs, rivaroxaban has accumulated the largest evidence base for the treatment of CAT, including having the only NOAC studies dedicated to treatment satisfaction and treatment persistence in patients with CAT.13
A Mayo Clinic study measured treatment duration when patients were treated with NOACs versus LMWHs. Patients receiving rivaroxaban had a longer mean treatment duration of 7.5 months versus 5.5 months with enoxaparin. Patients were also more likely to persist with their treatment beyond 6 months compared with those receiving enoxaparin (43.1% [113/262] versus 26.5% [131/494]).14
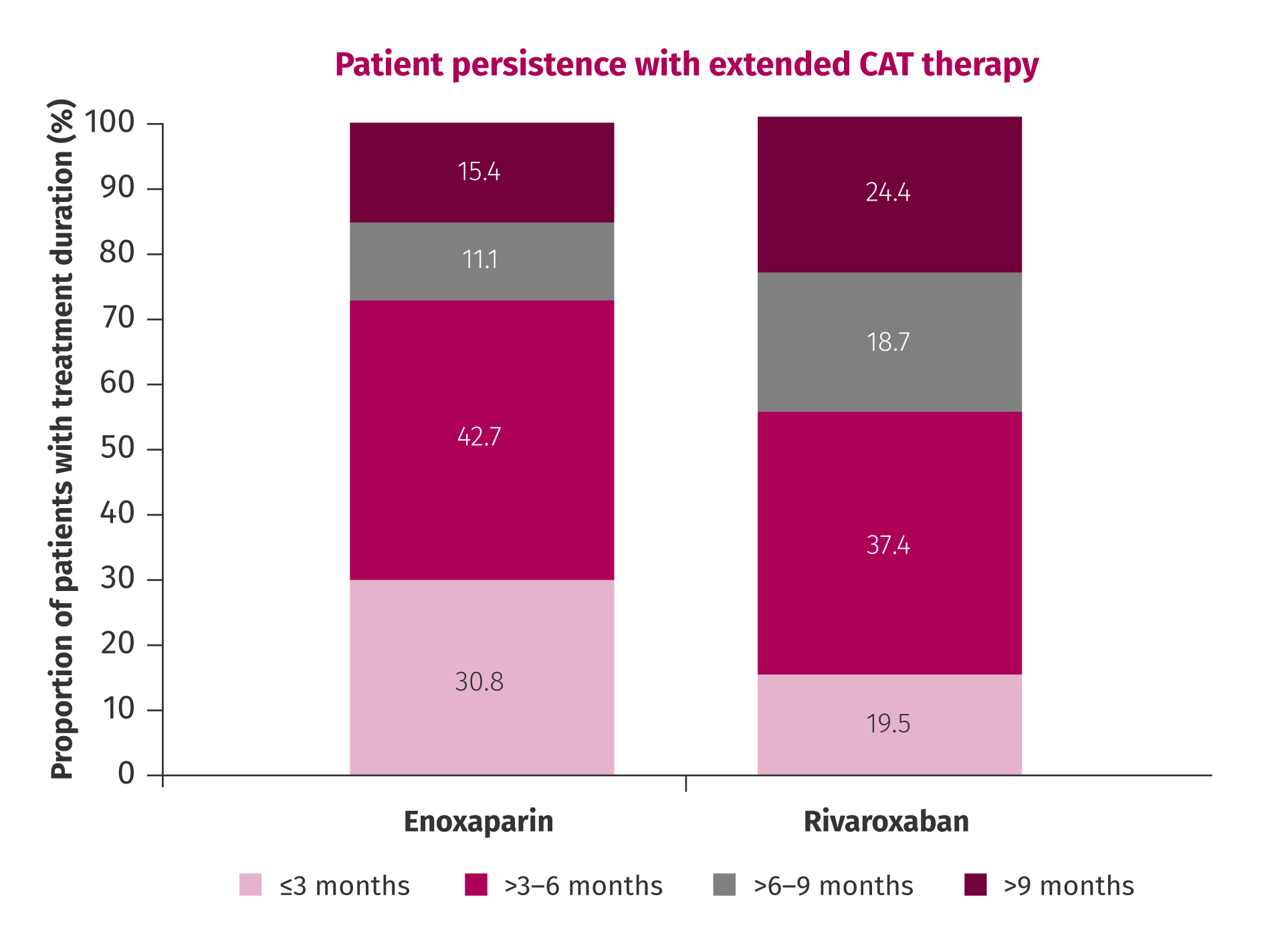
Persistence of patients with CAT treated with rivaroxaban or enoxaparin for an extended duration.
One of the most recent studies from the global, multi-study CALLISTO programme, CONKO‑011, compares real-life use and patient satisfaction with rivaroxaban versus LMWH in patients with CAT.15 Results demonstrated that patients receiving rivaroxaban were less likely to request early cessation of treatment than those receiving LMWHs up to 3 months.16 Patients consistently reported a lower relative anticoagulation-related burden with rivaroxaban than with LMWH over a 3 months period, and relative benefits favouring rivaroxaban up to 2 months.16

Fewer patients treated with rivaroxaban requested early cessation of therapy compared with LMWHs.
LMWH, low molecular weight heparin.
The findings from CONKO-011 and the Mayo Clinic study are supported by data from several previous studies. It has been shown that 61% of patients with CAT receiving rivaroxaban remain on the same therapy after 6 months, compared with 37% of patients initially receiving LMWH.17 Patients with CAT also reported improved quality of life and treatment satisfaction after switching from traditional anticoagulation therapy to rivaroxaban in the COSIMO study.13
Conclusion
When treating patients with CAT, it is necessary to evaluate the benefits and risks of extended anticoagulation in each individual patient. Patient-centric decision making should also consider the implications of treatment choice on adherence, persistence and quality of life to best protect your patients, like Claire.
References
- Fernandes CJ, Morinaga LTK, Alves Jr JL et al. Cancer-associated thrombosis: the when, how and why. European Respiratory Review 2019;28. Return to content
- Khorana A, McCrae K, Milentijevic D et al. Duration of anticoagulant therapy and VTE recurrence in patients with cancer. J Clin Oncol 2016;34. Abstract 10112. Return to content
- Qureshi W, Ali Z, Amjad W et al. Venous thromboembolism in cancer: an update of treatment and prevention in the era of newer anticoagulants. Front Cardiovasc Med 2016;3:24. Return to content
- Prandoni P, Lensing AWA, Piccioli A et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100:3484–3488. Return to content
- Konstantinides SV, Meyer G, Becattini C et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543–603. Return to content
- Key NS, Khorana AA, Kuderer NM et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2020;38:496–520. Return to content
- Farge D, Frere C, Connors JM et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2019;20:e566–e581. Return to content
- Streiff MB, Holmstrom B, Angelini D et al. NCCN guidelines insights: Cancer-associated venous thromboembolic disease, version 2.2018. J Natl Compr Canc Netw 2018;16:1289–1303. Return to content
- Lyman GH, Carrier M, Ay C et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv 2021;5:927–974. Return to content
- Streiff M, Abutalib S, Farge D et al. Update on Guidelines for the Management of Cancer-Associated Thrombosis. Oncologist 2021;26:e24–e40. Return to content
- Munoz Martin AJ, Gallardo Diaz E, Garcia Escobar I et al. SEOM clinical guideline of venous thromboembolism (VTE) and cancer (2019). Clin Transl Oncol 2020;22:171–186. Return to content
- Wojtukiewicz MZ, Skalij P, Tokajuk P et al. Direct oral anticoagulants in cancer patients. Time for a change in paradigm. Cancers (Basel) 2020;12. Return to content
- Picker N, Lee AY, Cohen AT et al. Anticoagulation treatment in cancer-associated venous thromboembolism: assessment of patient preferences using a discrete choice experiment (COSIMO Study). Thromb Haemost 2021;121:206–215. Return to content
- Houghton DE, Vlazny DT, Casanegra AI et al. Bleeding in patients with gastrointestinal cancer compared with nongastrointestinal cancer treated with apixaban, rivaroxaban, or enoxaparin for acute venous thromboembolism. Mayo Clin Proc 2021;96:2793–2805. Return to content
- Riess H, Sinn M, Kreher S, für den Arbeitskreis Hämostaseologie der Deutschen Gesellschaft für Hämatologie und Medizinische Onkologie (DGHO). CONKO-011: Evaluation of patient satisfaction with the treatment of acute venous thromboembolism with rivaroxaban or low molecular weight heparin in cancer patients. A randomized phase III study. Dtsch Med Wochenschr 2015;140(Suppl 1):S22–S23. Return to content
- Riess H, Sinn M, Lohneis A et al. Improved patient-reported treatment satisfaction with rivaroxaban as compared to low molecular weight heparins for cancer patients with acute venous thromboembolism - results from the CONKO-011 trial. ISTH. Virtual, 17–21 July 2021. ePoster LPB0041. Available at: https://isth2021.abstractserver.com/program/#/details/presentations/2426 [accessed 17 December 2021]. Riess H, Sinn M, Lohneis A et al. Improved patient-reported treatment satisfaction with rivaroxaban as compared to low molecular weight heparins for cancer patients with acute venous thromboembolism - results from the CONKO-011 trial. ISTH. Virtual, 17–21 July 2021. ePoster LPB0041. Available at: https://isth2021.abstractserver.com/program/#/details/presentations/2426 [accessed 17 December 2021]. Return to content
- Khorana AA, McCrae KR, Milentijevic D et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res Pract Thromb Haemost 2017;1:14–22. Return to content






