Protecting vulnerable patients with VTE: Choose a guideline-recommended therapy that has been thoroughly tested

Ken is 68 years old and has played the guitar in a band for over 15 years. Five years ago, Ken found himself having difficulty sleeping and urinating more frequently during the night. As these symptoms persisted, he began to lose his appetite and regularly found himself short of breath. After a visit to his doctor, urine and blood tests confirmed that Ken had abnormal kidney function with a creatinine clearance (CrCl) of 46 ml/min.
The band recently completed a national tour, travelling in a tour bus to more than 30 cities over 50 dates. Towards the end of the tour, Ken noticed that his right leg had become swollen and felt painful to the touch. Due to an extended period of immobility, he had developed a deep vein thrombosis (DVT).
What does the data tell us about treating patients with abnormal kidney function for acute venous thromboembolism (VTE)?
Considering individual patient needs when providing guideline-recommended protection
Guidelines suggest the use of non-vitamin K antagonist oral anticoagulants (NOACs) over vitamin K antagonists (VKAs) in patients with VTE for the first 3 months of treatment.1-3
Some patients are at greater risk of venous thromboembolic events and, therefore, require special attention. For example, patients who have moderately reduced kidney function (estimated glomerular filtration rate 30–60 ml/min/1.73 m2) are more than twice as likely to suffer a VTE than patients with normal kidney function.4 However, these patients can be challenging to treat because of their increased risk of bleeding during anticoagulation therapy.4-6

Vulnerable patients require guideline-recommended anticoagulation that has been thoroughly tested in patients like them. So how can you ensure that your vulnerable patients receive the validated protection that they deserve?
Vulnerable patients need a treatment that has been thoroughly tested in patients like them
Phase III trials have investigated the efficacy and safety of NOACs for the treatment of acute VTE, but the patient populations were different in each trial.7-11 Across all NOAC VTE trials, the EINSTEIN programme recruited the greatest number of patients with CrCl 30–≤50 ml/min.9-12
Outcomes in patients with moderately impaired kidney function were reassuring. In a pre-specified pooled subgroup analysis of the EINSTEIN-DVT and EINSTEIN-PE studies, rivaroxaban was non-inferior to low molecular weight heparin (LMWH)/VKA for the treatment of DVT or PE and demonstrated protection from VTE recurrence across kidney function thresholds without increasing their risk of major bleeding compared with LMWH/VKA.12

Incidence of recurrent VTE and major bleeding across kidney function thresholds in the pooled analysis of EINSTEIN-DVT and EINSTEIN-PE trials.12
CrCl, creatinine clearance; LMWH, low molecular weight heparin; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Furthermore, rivaroxaban was investigated in the prospective, international, non-interventional study, XALIA, which also included a high proportion of vulnerable patients.13 Notably, the consistent benefit in protection from recurrent VTE and major bleeding events with rivaroxaban was also observed in real-world practice in patients with DVT in XALIA.13
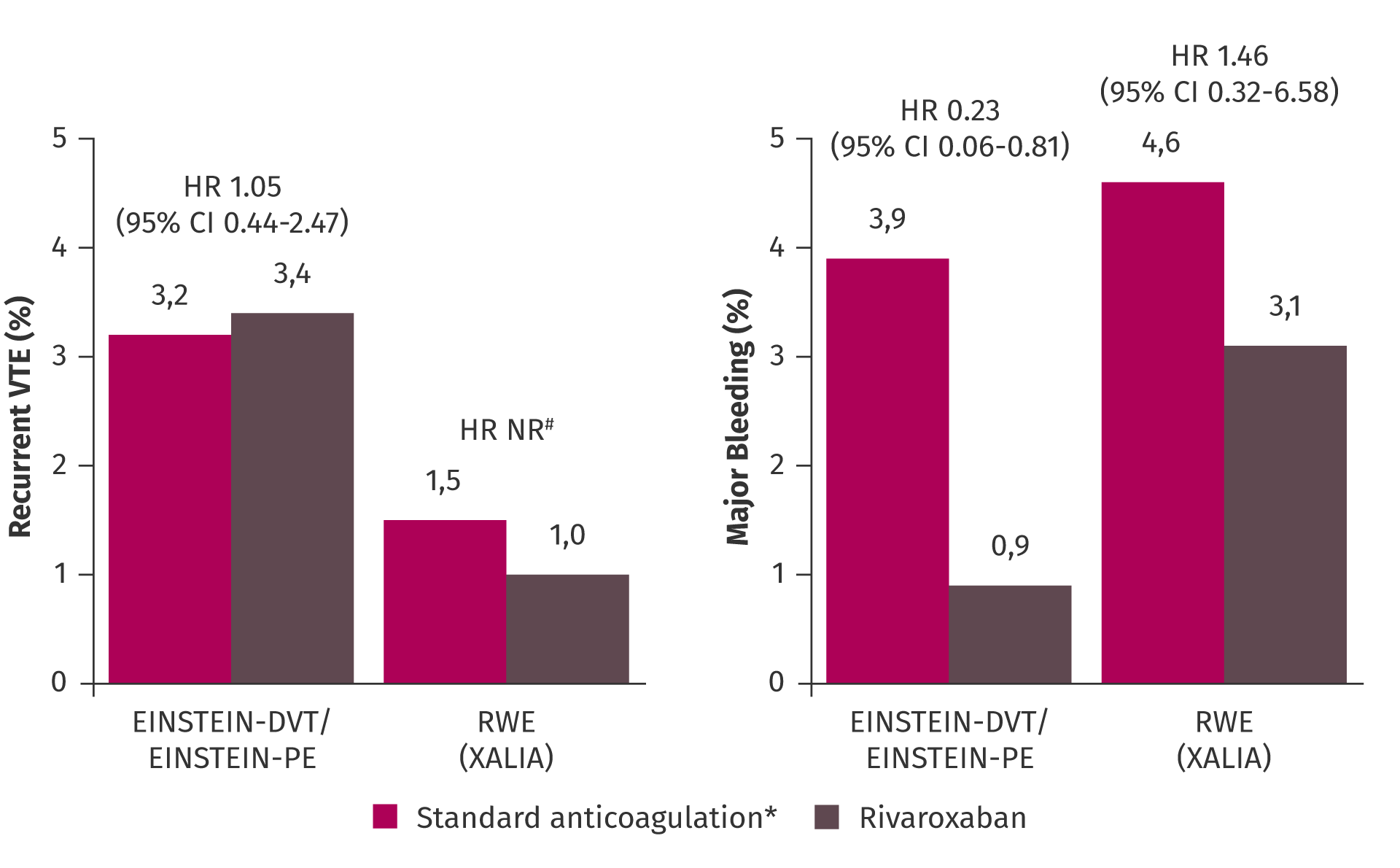
Efficacy and safety of rivaroxaban versus standard anticoagulation in patients with DVT or PE and moderately reduced GFR.12,13
Patients with CrCl 30–49 ml/min in EINSTEIN-DVT/EINSTEIN-PE and <50 ml/min in XALIA.
*LMWH followed by VKA in EINSTEIN-DVT/EINSTEIN-PE; UFH, LMWH or fondaparinux followed by VKA in XALIA.
#HR could not be calculated due to the low number of events.
CI, confidence interval; CrCl, creatinine clearance; GFR, glomerular filtration rate; HR, hazard ratio; LMWH, low molecular weight heparin; NR, not reported; RWE, real-world evidence; UFH, unfractionated heparin; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Protecting your patients with VTE, from babies to grandparents
The pooled analysis of EINSTEIN-PE and EINSTEIN-DVT also provided high quality evidence in fragile patients with VTE who are at high risk of recurrent events and major bleeding.14-16 Fragile patients were defined as patients with one or more of >75 years of age, CrCl <50 ml/min or low body weight (≤50 kg). Rivaroxaban-treated fragile patients had a significantly lower risk of major bleeding compared with patients treated with LMWH/VKA. Furthermore, a similar level of protection from recurrent VTE events was observed with rivaroxaban compared with LMWH/VKA irrespective of fragility.17

Incidence of recurrent VTE and major bleeding in fragile and non-fragile patients in the pooled analysis of the EINSTEIN-DVT and EINSTEIN-PE trials.17
*ITT population (N=8281); fragile patients (n=1573).
#One or more of: >75 years old, CrCl <50 ml/min, low body weight (≤50 kg).
‡Safety population (N=8246); fragile patients (n=1567).
ARR, absolute risk reduction; ITT, intention to treat; LMWH, low molecular weight heparin; RRR, relative risk reduction; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Rivaroxaban has a breadth of extensive clinical evidence demonstrating consistent efficacy and safety results in a broad range of patient populations
Rivaroxaban has been thoroughly tested in patients with VTE and abnormal kidney function, like Ken. The EINSTEIN-DVT and EINSTEIN-PE pooled analysis and the real-world XALIA study have demonstrated a favourable efficacy profile for rivaroxaban across vulnerable patient subgroups, including fragile patients. The reassuring safety data from these studies suggest that you can be confident when treating patients whose comorbidities put them at an increased risk of major bleeding. So, when considering how to best protect your vulnerable patients from VTE recurrence, choose guideline-recommended protection that has a breadth of evidence in patients like them.
References
- Kearon C, Akl EA, Ornelas J et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–352. Return to content
- Konstantinides SV, Meyer G. The 2019 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2019;40:3453–3455. Return to content
- Ortel TL, Neumann I, Ageno W et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 2020;4:4693–4738. Return to content
- Ocak G, Lijfering WM, Verduijn M et al. Risk of venous thrombosis in patients with chronic kidney disease: identification of high-risk groups. J Thromb Haemost 2013;11:627–633. Return to content
- Monreal M, Falgá C, Valle R et al. Venous thromboembolism in patients with renal insufficiency: findings from the RIETE Registry. Am J Med 2006;119:1073–1079. Return to content
- Grand'Maison A, Charest AF, Geerts WH. Anticoagulant use in patients with chronic renal impairment. Am J Cardiovasc Drugs 2005;5:291–305. Return to content
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–2510. Return to content
- The EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–1297. Return to content
- Agnelli G, Buller HR, Cohen A et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808. Return to content
- The Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–1415. Return to content
- Goldhaber SZ, Schulman S, Eriksson H et al. Dabigatran versus warfarin for acute venous thromboembolism in elderly or impaired renal function patients: pooled analysis of RE-COVER and RE-COVER II. Thromb Haemost 2017;117:2045–2052. Return to content
- Bauersachs RM, Lensing AWA, Prins MH et al. Rivaroxaban versus enoxaparin/vitamin K antagonist therapy in patients with venous thromboembolism and renal impairment. Thromb J 2014;12:25–32. Return to content
- Ageno W, Mantovani LG, Haas S et al. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): an international, prospective, non-interventional study. Lancet Haematology 2016;3:e12–e21. Return to content
- Damanti S, Braham S, Pasina L. Anticoagulation in frail older people. J Geriatr Cardiol 2019;16:844–846. Return to content
- Moustafa F, Giorgi Pierfranceschi M, Di Micco P et al. Clinical outcomes during anticoagulant therapy in fragile patients with venous thromboembolism. Res Pract Thromb Haemost 2017;1:172–179. Return to content
- Bucherini EH-B, L. M., Fernandez-Capitan CL, A., Villalobos A et al. Fragile patients with venous thrombembolism, what did we learn from RIETE. Int J Hematol Res 2015;1:35–39. Return to content
- Prins MH, Lensing AWA, Bauersachs R et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J 2013;11:21. Return to content






