Treatment and Prevention of Recurrent Events
An overview of the treatment and prevention of cancer-associated thrombosis
In this section:
- Effective treatment of VTE and protection against recurrence
- Choice of anticoagulant
- The evolution of anticoagulant choice available for cancer-associated thrombosis
- Clinical evidence on NOAC use for the treatment of CAT
- Guideline recommendations
- Duration of therapy
- Clinical evidence
- Guideline recommendations
- Why patient-centric decision-making matters
- Clinical evidence
Effective treatment of VTE and protection against recurrence
Fast and effective anticoagulation is essential for preventing fatalities and mitigating the risk of venous thromboembolism (VTE) recurrence.1
Inadequate use of anticoagulants, in respect to dose or duration, may result in recurrent events that were otherwise avoidable.2

Relative increase in the rate of recurrent VTE and major bleeding events in patients with cancer compared with those without cancer
Several studies have demonstrated that mortality rates for recurrent VTE are higher than those for bleeding events.3
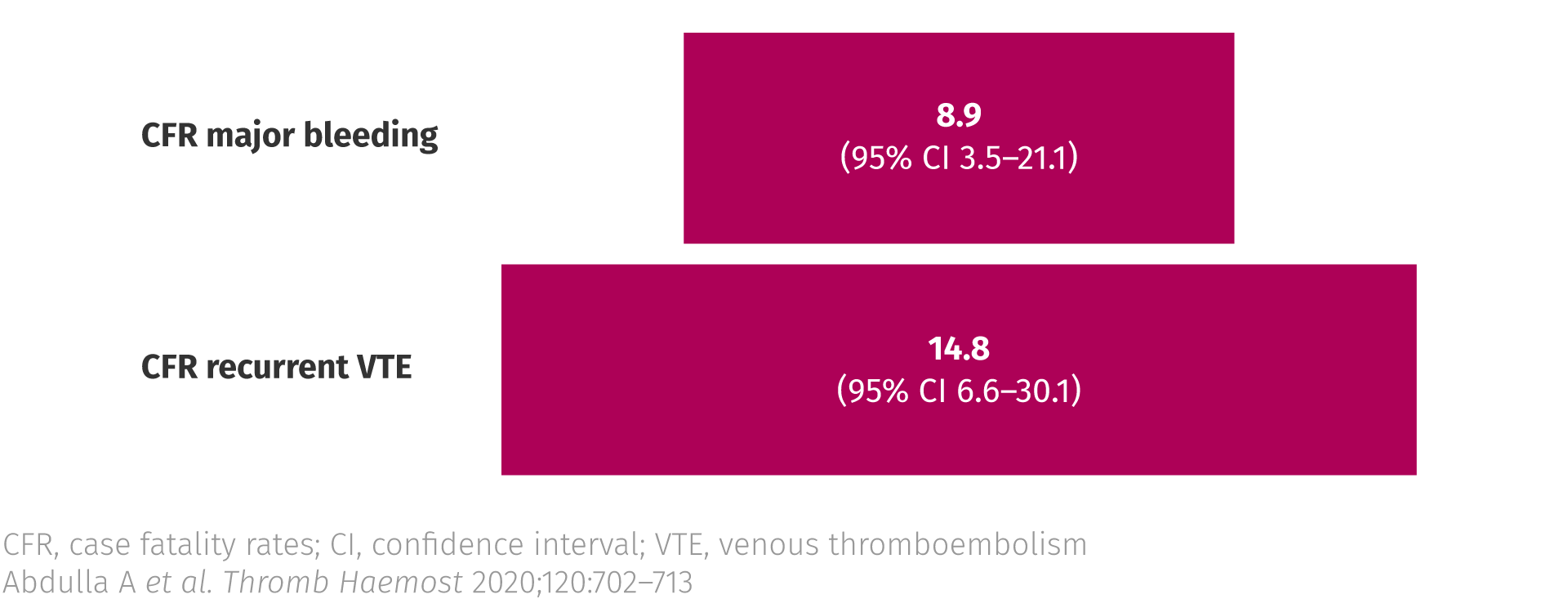
Case fatality rates from recurrent thrombosis and major bleeding in patients with cancer3
Choice of anticoagulant
The evolution of anticoagulant choice available for cancer-associated thrombosis
In the evolution of anticoagulant choice available for cancer-associated thrombosis (CAT), two major changes have taken place: 1) the move from vitamin K antagonists (VKAs) to low molecular weight heparins (LMWHs) as the guideline-preferred standard of care; 2) the endorsement of non-vitamin K antagonist oral anticoagulant (NOACs) as a treatment option in this setting.4-7
VKAs were the mainstay of treatment for VTE in patients with and without cancer for many years, but alternatives were warranted due to challenges unique to VKA therapy, including:
- The need for frequent monitoring and dose adjustment to maintain the drug effect within a narrow therapeutic window8,9
- Issues with both food–drug and drug–drug interactions (including with anti-cancer agents and supportive therapies)8,9
Over a decade ago, LMWHs replaced VKAs as the standard of care for the treatment of CAT based on:
- The overall beneficial efficacy and safety profile of LMWHs compared with VKAs in patients with CAT, as demonstrated by several randomized controlled trials (RCTs)5
- The suitability of LMWHs for patients who have difficulty with oral therapy (e.g. due to vomiting) and ease of management compared with VKAs in cases of invasive interventions or thrombocytopenia5
However, there are challenges associated with the use of LMWHs, these include:
These factors may impact on persistence with therapy,10 as demonstrated by lower levels of persistence with LMWHs than with oral anticoagulants in various studies.11,12
More recently, the NOACs edoxaban and rivaroxaban13-16 (and in most recent updates apixaban)7 have been endorsed by several international clinical guidelines for the treatment of CAT based on head-to-head comparisons with the LMWH dalteparin in RCTs.12,17-19
Clinical evidence on NOAC use for the treatment of CAT
To date there have been four RCTs comparing NOACs with dalteparin for the treatment of CAT: Hokusai-VTE-Cancer12 for edoxaban, SELECT-D17 for rivaroxaban, and ADAM-VTE and CARAVAGGIO for apixaban.18,19
A reduction in the risk of recurrent VTE with NOAC therapy compared with dalteparin was observed across all trials.12,17-19 This came at the cost of a significant increase in major bleeding with edoxaban and rivaroxaban in Hokusai-VTE-Cancer and SELECT-D, respectively.12,17 A high proportion of major bleeding events with NOAC therapy were gastrointestinal12,17,19 and occurred in patients with primary gastrointestinal tumours.12,17 The incidence of fatal bleeding events was similar between treatment arms across all trials.17-21

Meta-analysis of data from Hokusai-VTE-Cancer, SELECT-D, ADAM-VTE and CARRAVAGIO comparing the relative risks of VTE recurrence and major bleeding at 6 months with NOAC versus LMWH therapy for the treatment of CAT21
Results from an additional RCT, CASTA-DIVA, in which the efficacy and safety of rivaroxaban for the treatment of CAT in patients with a high risk of VTE recurrence were investigated, are awaiting publication.22,23
In addition to RCTs, there is accumulating real-world evidence on the use of NOACs for the treatment of CAT (specifically for rivaroxaban and apixaban), which provides some reassurance on the effectiveness and safety of NOACs in this clinical setting. For example:
- The COSIMO study collected data on clinical outcomes in 505 patients with active cancer who had had switched to rivaroxaban following ≥4 weeks on LMWH/VKA treatment for VTE.24 The observed rates of recurrent VTE and bleeding events were similar to those in SELECT-D, Hokusai-VTE-Cancer and CARAVAGGIO12,17,19,24
- In a prospective cohort study conducted at the Mayo Thrombophilia Clinic, the risk of recurrent VTE and major bleeding was similar between patients treated with apixaban, rivaroxaban and a LMWH. Rivaroxaban had a significantly higher rate of non-major bleeding compared with apixaban, whilst the mortality rate was lower with rivaroxaban than both apixaban and LMWH therapy25
Guideline recommendations
Updated clinical guidelines on the treatment of CAT include NOACs as a recommend treatment option (specifically edoxaban and rivaroxaban in updates predating publication of the apixaban trials).7,13-16 When choosing between a NOAC and LMWH, careful consideration of tumour type, risk factors for bleeding, potential for drug–drug interactions, and patient preference are recommended.
In general, NOACs are the preferred option for patients with CAT at a low risk of bleeding, with LMWHs preferred for those at a high risk of bleeding (including patients with upper gastrointestinal cancer).7,13-16
Considering that clinical circumstances can rapidly change for a patient with cancer, periodic reassessment of the risks and benefits of anticoagulation is crucial.5,26

Duration of therapy
Clinical evidence
In the absence of robust RCT data, current guideline recommendations regarding the duration of therapy for CAT are based on limited evidence.
Observations to date suggest that the risk of recurrent VTE remains higher than the risk of major bleeding beyond 6 months from the index event:
- In the DALTECAN and TiCAT studies, the incidences of recurrent VTE with LMWH between months 7–12 (4.1 and 1.1%, respectively) were similar to the incidences observed between months 2–6. In both studies, the rate of major bleeding remainedlow after the first month of therapy (0.5–0.7% between months 7–12)27,28
- In an extended phase of the SELECT-D study, patients who had completed 6 months of anticoagulation therapy and were deemed to have a high risk of recurrence (based on having an index pulmonary embolism event or residual vein thrombosis) were randomized to receive placebo or rivaroxaban. The absolute difference in the risk of recurrent VTE was reduced by 2.2% with rivaroxaban compared with placebo, whereas the absolute risk of major bleeding was increased by 0.8%. The study was insufficiently powered to detect differences between the treatment arms29
Of note, in the Hokusai-VTE-Cancer trial a similar trade-off between the benefits and risks of anticoagulation with edoxaban and dalteparin was observed in terms of outcomes between 6 and 12 months.30
Guideline recommendations
Recent guidelines recommend that extended anticoagulation therapy (beyond the initial 6 months) should be considered for an ‘indefinite period’ in patients with active cancer,7,10,14 or until the cancer is cured.16 Termination of anticoagulation therapy should be determined based on individual patient evaluation of the risk of bleeding, cancer activity, life expectancy, quality of life and patient preference.5,7,10,14-16

Why patient-centric decision-making matters
Clinical evidence
Patient-centric decision-making is considering integral to anticoagulation management in international guidelines for the treatment of CAT.
The burdens and benefits of therapy, usually centred around convenience of medication (e.g. considering route of adminstration and dosing schedule) among other factors, may impact on patient satisfaction and compliance with therapy (i.e. adherence and persistance), which in turn can impact on clinical outcomes.31-33

Convenience of an anticoagulant has the potential to impact on clinical outcomes
Of note, persistence with LMWH has been demonstrated to be lower than with oral anticoagulants, possibly because of the burden of daily injections and the high cost of therapy.5,11,12

Persistence with anticoagulant therapies for the treatment of CAT34
Results from patient-directed surveys designed to assess the percieved benefits and burdens of treatment options can be helpful to inform decision-making.
Patient-reported outcomes associated with anticoagulant use for the treatment of CAT have been evaluated in several studies:
- In the COSIMO study, patient-reported outcomes in patients switching from a LMWH or VKA to rivaroxaban for the treatment of CAT were evaluated and a clinically significant increase in treatment satisfaction was observed35
- Patients that took part in a discrete choice experiment during the COSIMO study reported a preference for oral over injectable therapy36
- Patient satisfaction favoured rivaroxaban and apixaban in head-to-head comparison with LMWH in the SELECT-D and ADAM-VTE trials, respectively18,37
- The ongoing randomized open-label study CONKO-011 is investigating patient satisfaction with rivaroxaban or LMWH in patients with acute CAT38
References
- Coleman R, Maccallum P. Treatment and secondary prevention of venous thromboembolism in cancer. Br J Cancer 2010;102(Suppl 1):S17–S23. Return to content
- Vasanthamohan L, Boonyawat K, Chai-Adisaksopha C, Crowther M. Reduced-dose direct oral anticoagulants in the extended treatment of venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost 2018;16:1288–1295. Return to content
- Abdulla A, Davis WM, Ratnaweera N et al. A meta-analysis of case fatality rates of recurrent venous thromboembolism and major bleeding in patients with cancer. Thromb Haemost 2020;120:702–713. Return to content
- Lyman GH, Bohlke K, Khorana AA et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol 2015;33:654-656. Return to content
- Kearon C., Akl E.A., Ornelas J. et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149(2):315-52. Return to content
- Farge D, Bounameaux H, Brenner B et al. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2016;17:e452-e466. Return to content
- National Comprehensive Cancer Network. Cancer-associated venous thromboembolic disease, Version 1.2020. National Comprehensive Cancer Network, Inc. 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf [accessed 6 May 2020]. National Comprehensive Cancer Network. Cancer-associated venous thromboembolic disease, Version 1.2020. National Comprehensive Cancer Network, Inc. 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf [accessed 6 May 2020]. Return to content
- Riechelmann RP, Del Giglio A. Drug interactions in oncology: how common are they? Ann Oncol 2009;20:1907–1912. Return to content
- Bach M and Bauersachs R. Thromb Haemost. 2016;116:S24–S32. Return to content
- Khorana AA, Carrier M, Garcia DA, Lee AYY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis 2016;41:81-91. Return to content
- Khorana AA, McCrae KR, Milentijevic D et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res Pract Thromb Haemost 2017;1:14–22. Return to content
- Raskob G.E., van Es N., Verhamme P. et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2017;378(7):615–24. Return to content
- Khorana A.A., Noble S., Lee A.Y.Y. et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(9):1891–4. Return to content
- Key NS, Khorana AA, Kuderer NM et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2019;38:496–520. Return to content
- Farge D, Frere C, Connors JM et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2019;20:e566–e581. Return to content
- Konstantinides SV, Meyer G. The 2019 ESC guidelines on the Diagnosis and Management of Acute Pulmonary Embolism. Eur Heart J 2019;40:3453–3455. Return to content
- Young AM, Marshall A, Thirlwall J et al. Comparison of an oral Factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018;36:2017–2023. Return to content
- McBane RD, 2nd, Wysokinski WE, Le-Rademacher JG et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost 2020;18:411–421. Return to content
- Agnelli G, Becattini C, Meyer G et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med 2020;382:1599–1607. Return to content
- Mulder FI, Bosch FTM, Young AM et al. Direct oral anticoagulants for cancer-associated venous thromboembolism: a systematic review and meta-analysis. Blood 2020: doi:10.1182/blood.2020005819. Mulder FI, Bosch FTM, Young AM et al. Direct oral anticoagulants for cancer-associated venous thromboembolism: a systematic review and meta-analysis. Blood 2020: doi:10.1182/blood.2020005819. Return to content
- Giustozzi M, Agnelli G, Del Toro-Cervera J et al. Direct oral anticoagulants for the treatment of acute venous thromboembolism associated with cancer: a systematic review and meta-analysis. Thromb Haemost 2020: doi:10.1055/s-0040-1712098. Giustozzi M, Agnelli G, Del Toro-Cervera J et al. Direct oral anticoagulants for the treatment of acute venous thromboembolism associated with cancer: a systematic review and meta-analysis. Thromb Haemost 2020: doi:10.1055/s-0040-1712098. Return to content
- Assistance Publique – Hôpitaux de Paris. Cancer associated thrombosis, a pilot treatment study using rivaroxaban (CASTA-DIVA). 2018. Available at: https://clinicaltrials.gov/ct2/show/NCT02746185 [accessed 13 May 2020]. Assistance Publique – Hôpitaux de Paris. Cancer associated thrombosis, a pilot treatment study using rivaroxaban (CASTA-DIVA). 2018. Available at: https://clinicaltrials.gov/ct2/show/NCT02746185 [accessed 13 May 2020]. Return to content
- Elalamy I, Mahe I, Ageno W, Meyer G. Long-term treatment of cancer-associated thrombosis: the choice of the optimal anticoagulant. J Thromb Haemost 2017;15:848–857. Return to content
- Maraveyas A, Beyer-Westendorf J, Lee AYY et al. Baseline characteristics and clinical outcomes from the cancer associated thrombosis - patient reported outcomes with rivaroxaban (COSIMO) Trial. Blood 2019;134:2161. Return to content
- Wysokinski WE, Houghton DE, Casanegra AI et al. Comparison of apixaban to rivaroxaban and enoxaparin in acute cancer-associated venous thromboembolism. Am J Hematol 2019;94:1185–1192. Return to content
- Lee AYY. When can we stop anticoagulation in patients with cancer-associated thrombosis? Blood 2017;130:2484–2490. Return to content
- Francis CW, Kessler CM, Goldhaber SZ et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemost 2015;13:1028-1035. Return to content
- Jara-Palomares L, Solier-Lopez A, Elias-Hernandez T et al. Tinzaparin in cancer associated thrombosis beyond 6 months: TiCAT study. Thromb Res 2017;157:90–96. Return to content
- Marshall A, Levine M, Hill C et al. Treatment of cancer-associated venous thromboembolism: 12-month outcomes of the placebo versus rivaroxaban randomization of the SELECT-D Trial (SELECT-D: 12m). J Thromb Haemost 2020;18:905–915. Return to content
- Di Nisio M, van Es N, Carrier M et al. Extended treatment with edoxaban in cancer patients with venous thromboembolism: A post-hoc analysis of the Hokusai-VTE Cancer study. J Thromb Haemost 2019;17:1866–1874. Return to content
- Barbosa C.D., Balp M.M., Kulich K. et al. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence 2012;6:39-48. Barbosa C.D., Balp M.M., Kulich K. et al. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence 2012;6:39-48. Return to content
- Cohen AT, Maraveyas A, Beyer-Westendorf J et al. COSIMO - patients with active cancer changing to rivaroxaban for the treatment and prevention of recurrent venous thromboembolism: a non-interventional study. Thromb J 2018;16:21. Return to content
- Abdou JK, Auyeung V, Patel JP, Arya R. Adherence to long-term anticoagulation treatment, what is known and what the future might hold. Br J Haematol 2016;174:30–42. Return to content
- Khorana AA, Francis CW, Kuderer NM et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Thromb Res 2017;151:89–95. Return to content
- Cohen AT, Maraveyas A, Beyer-Westendorf J et al. Patient-reported outcomes associated with switching to rivaroxaban for the treatment of venous thromboembolism in patients with active cancer. European Society of Medical Oncology. Barcelona, Spain, 27 September–1 October 2019. Poster P1774P. Return to content
- Picker N, Cohen AT, Maraveyas A et al. Patient preferences regarding anticoagulation therapy in patients with cancer having a VTE event - a discrete choice experiment in the COSIMO study. 61st American Society of Hematology. Orlando, FL, USA, 7–10 December 2019 2019. Poster 2159. Return to content
- Hutchinson A, Rees S, Young A et al. Oral anticoagulation is preferable to injected, but only if it is safe and effective: An interview study of patient and carer experience of oral and injected anticoagulant therapy for cancer-associated thrombosis in the select-d trial. Palliat Med 2019;33:510–517. Return to content
- Riess H, Sinn M, Kreher S, für den Arbeitskreis Hämostaseologie der Deutschen Gesellschaft für Hämatologie und Medizinische Onkologie (DGHO). [CONKO-011: Evaluation of patient satisfaction with the treatment of acute venous thromboembolism with rivaroxaban or low molecular weight heparin in cancer patients. A randomized phase III study]. Dtsch Med Wochenschr 2015;140(Suppl 1):S22–S23. Return to content





