Managing Atherosclerosis
How are patients with coronary artery disease (CAD) currently managed?
CAD is a dynamic process, and lifestyle modifications, medication or surgical interventions can cause disease stabilization or regression. The disease is chronic and can have long clinically silent periods, but can also become unstable at any time, usually due to an acute event. To emphasize this, the 2019 European Society of Cardiology (ESC) guidelines introduced the term “chronic coronary syndromes” (CCS) to replace the older term “stable CAD”, which gave the impression that patients were at low risk of ischaemic events.1 Read the newsletter ‘From stable CAD to chronic coronary syndromes: Evolving terminology in cardiovascular disease’ to find out more about these changes in terminology.
For protection against major cardiovascular events in patients with CCS, the 2019 ESC guidelines recommend:1
- Single antiplatelet therapy in patients with a previous myocardial infarction (MI) or revascularization
- A statin to reduce blood low-density lipoprotein levels in all patients with CCS
- An antihypertensive if required
For long-term secondary prevention in patients with CCS, the 2019 ESC guidelines recommend:1
The 2019 ESC guideline recommendations for secondary prevention in patients with chronic coronary syndromes
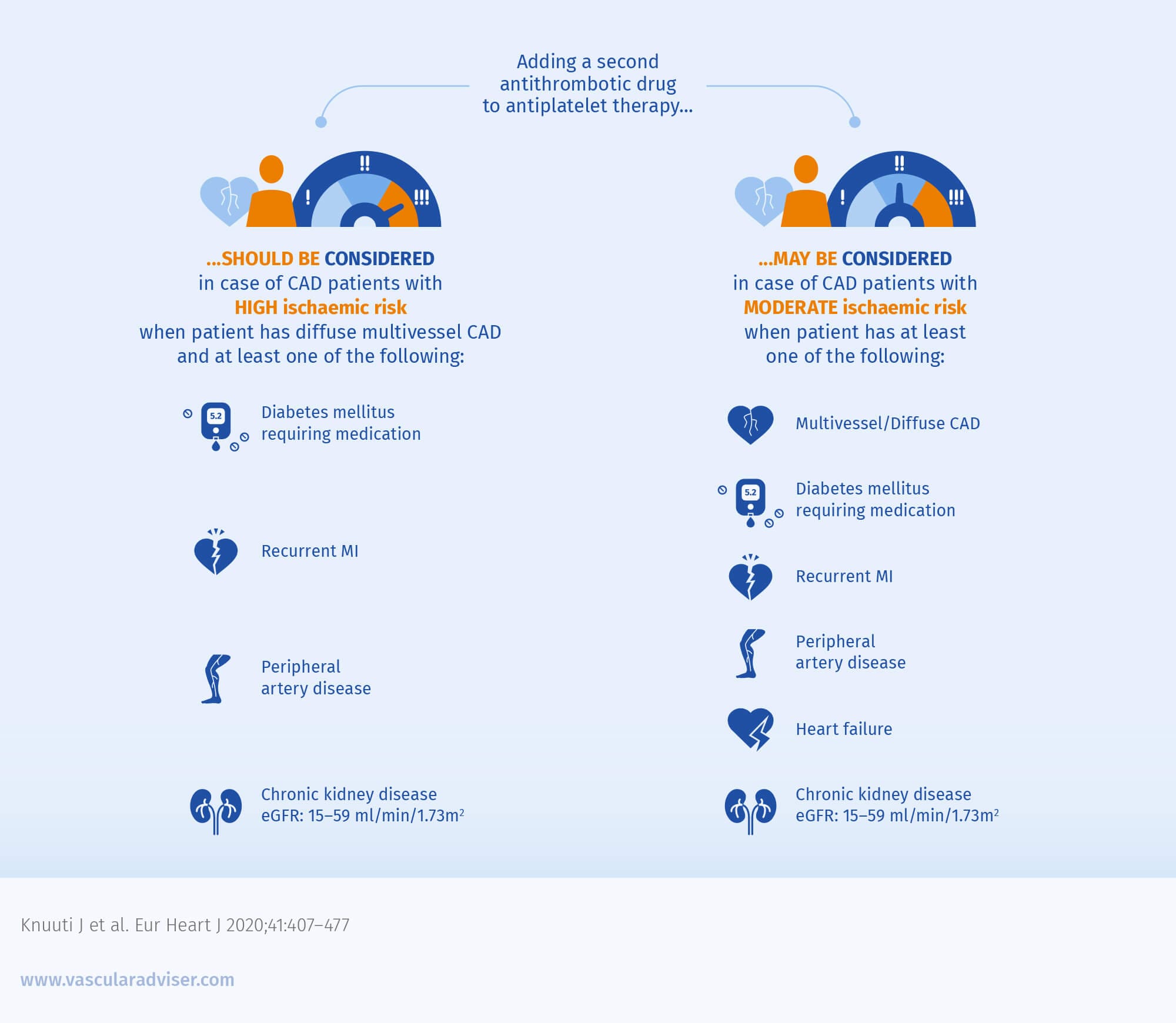
The 2019 ESC guidelines recommend considering adding a second antithrombotic drug to antiplatelet therapy in patients with moderate or high ischaemic risk
The 2019 ESC guidelines on diabetes, pre-diabetes and cardiovascular diseases also specify that adding a second antithrombotic drug should be considered in patients with CCS and diabetes.2
Both 2019 ESC guidelines specify that adding a second antithrombotic drug should only be considered in patients without high bleeding risk.1,2 The recommended options for the second antithrombotic drug include:1
- A second antiplatelet agent post MI and post percutaneous coronary intervention for MI in patients who have tolerated dual antiplatelet therapy for 1 year
- The COMPASS regimen post MI >1 year or in patients with multivessel CAD
Control of CAD symptoms involves the use of anti-ischaemic drugs to provide angina relief:1,3
- First-line therapies consist of short-acting nitrates to cause coronary vasodilatation, with beta-blockers and/or calcium channel blockers to control heart rate, increase perfusion to ischaemic areas and promote vasodilation
- Second-line therapies include long-acting nitrates and ranolazine
If symptoms persist despite optimal medical therapy, revascularization may be indicated to provide symptom relief in patients with major coronary stenosis.3,4
Professor Stuart Connolly discusses progress in CAD treatment.
How CAD treatment has changed
PP-XAR-ALL-2002-1
How are patients with peripheral artery disease (PAD) currently managed?
Management of patients with PAD involves the prevention of major thrombotic events and the control of symptoms. To prevent the thrombotic events, practice guidelines recommend a combination of risk factor management (lipid and blood pressure control) and antithrombotic therapy.2,5-8
Traditionally, antithrombotic therapy has taken the form of single antiplatelet therapy, recommended in both the 2016 American Heart Association/American College of Cardiology (AHA/ACC) guidelines and the 2017 European Society of Cardiology (ESC) guidelines.5,6 However, data from the recent COMPASS trial6,7 have led to the evolution of these guidelines. The 2019 Global Vascular Guidelines and the 2019 European Society for Vascular Medicine (ESVM) guidelines both suggest considering dual pathway inhibition for patients with symptomatic PAD and without a high risk of bleeding.7,8 For more details about DPI therapy, please see the What is dual pathway inhibition? section. Furthermore, the 2019 ESC guidelines on diabetes, pre-diabetes and cardiovascular diseases recommend considering dual pathway inhibition for diabetes patients with concomitant symptomatic PAD, and the 2019 ESC guidelines on chronic coronary syndromes suggest considering a second antithrombotic agent (such as the COMPASS regimen) in patients at high ischaemic risk, such as those with concomitant PAD.1,2
In addition to their thrombotic risk, patients with PAD often present with leg pain (e.g. intermittent claudication), and this can limit their daily activities and impact on their quality of life.9 The 2016 AHA/ACC guidelines, 2017 ESC guidelines and 2019 ESVM guidelines recommend a supervised and structured exercise programme together with medical therapies aimed at reducing limb symptoms by suppressing platelet aggregation and promoting vasodilation to manage the limb symptoms in patients with PAD.5-7 If no improvements in limb symptoms are seen after conservative therapy, revascularization may be considered.5-7
Professor John Eikelboom discusses progress in PAD treatment.
How PAD treatment has changed
PP-XAR-ALL-2134-1
When do patients with peripheral artery disease (PAD) need revascularization?
Lower extremity revascularization may relieve the symptoms of patients with PAD,7,12 but at a risk of post-operative complications such as surgical site infection and bleeding.13 Therefore, it is necessary to carefully evaluate if the benefits from revascularization outweigh the risks of the procedure.
Current practice guidelines provide an overview of patients who may benefit from revascularization. Based on the 2017 European Society of Cardiology (ESC) guidelines and the 2019 European Society of Vascular Medicine (ESVM) guidelines, revascularization should be considered for patients with intermittent claudication only when their daily lives are compromised despite exercise therapy.6,7 However, for patients with critical limb threatening ischaemia, the 2017 ESC guidelines, 2019 ESVM guidelines and 2019 Global Vascular Guidelines indicate revascularization whenever feasible to salvage the limb.6-8
What medications are needed following a peripheral revascularization procedure?
Following a peripheral revascularization procedure, patients are at a high risk of thrombotic events.14,15 Therefore, optimal antithrombotic therapy in these patients is essential.
Recent guidelines, however, vary in their recommended antithrombotic treatment after peripheral revascularization. The medication options after surgical revascularization include single antiplatelet therapy (2017 European Society of Cardiology [ESC] guidelines and 2019 European Society for Vascular Medicine [ESVM] guidelines), vitamin K antagonists (2017 ESC guidelines and 2019 ESVM guidelines) and dual antiplatelet therapy (DAPT) for specific cases of below the knee prosthetic bypass grafting (2017 ESC guidelines and 2019 ESVM guidelines) or prosthetic bypass surgery (2019 Global Vascular Guidelines).6,8
After endovascular revascularization, the guidelines suggest antiplatelet therapy. The 2017 ESC guidelines and 2019 ESVM guidelines suggest long-term single antiplatelet therapy after peripheral endovascular revascularization.6,7 The 2017 ESC guidelines, 2019 ESVM guidelines and 2019 Global Vascular Guidelines recommend considering DAPT in patients after endovascular revascularization.6,7,16 The 2016 American Heart Association/American College of Cardiology (AHA/ACC) guideline on the management of patients with lower extremity peripheral artery disease (PAD) also suggest DAPT in patients with symptomatic PAD after revascularization.5 However, the recommendations on DAPT for the prevention of thrombotic events after peripheral endovascular revascularization are based on clinical judgement and consensus because the data from clinical trials is limited.5-8 This shows that there is a lack of data based evidence for the efficiency of DAPT for preventing thrombotic events in patients with symptomatic PAD.
More recently, the VOYAGER PAD clinical trial demonstrated that dual pathway inhibition can significantly reduce major adverse limb events in patients with symptomatic PAD following revascularization without a significant increase in major bleeding, offering a new therapeutic approach in this clinical setting.17
For more information, please see How can outcomes be improved in patients with symptomatic peripheral artery disease (PAD)?
What is dual pathway inhibition?
Two pathways contribute to arterial thrombosis: platelet activation and the activation of the coagulation cascade.18 Dual pathway inhibition is an antithrombotic regimen composed of a low-dose anticoagulant and an antiplatelet. This combination targets both pathways to reduce the risk of cardiovascular events.18,19

Dual pathway inhibition targets platelet activation and the activation of coagulation cascade
The mechanism of action of dual pathway inhibition
The evidence for the benefit of dual pathway inhibition comes from the COMPASS study, which enrolled 27,395 patients with stable atherosclerotic disease who were already receiving a high standard of treatment.9
The COMPASS regimen was associated with a significant 24% reduction in the composite risk of myocardial infarction, stroke and cardiovascular death compared with an antiplatelet alone.9
As would be expected given the use of an additional antithrombotic, the COMPASS regimen was associated with a significant increase in major bleeding compared with an antiplatelet alone, although there were no significant differences in the risks of intracranial haemorrhage or fatal bleeding.9
The benefit of the COMPASS regimen was found to be especially high in patients with coronary artery disease and/or peripheral artery disease and high vascular risk, including patients with polyvascular disease, heart failure, renal insufficiency (estimated glomerular filtration rate 15–60 ml/min) or diabetes.20 More information can be found in the risk stratification newsletter.
Additional support for the efficacy and safety of dual pathway inhibition comes from the VOYAGER PAD study, which enrolled patients with symptomatic peripheral artery disease undergoing revascularization procedures. In this patient population, dual pathway inhibition significantly reduced limb ischaemia and major cardiovascular events in comparison to antiplatelet therapy. Additionally, TIMI major bleeding did not significantly increase with dual pathway inhibition compared with antiplatelet therapy.17
The COMPASS trial explained.
Video explaining the results of the COMPASS trial
PP-XAR-ALL-0516-2
The VOYAGER PAD trial explained.
Video explaining the results of the VOYAGER PAD trial
PP-M_RIV-ALL-0109-1
How can outcomes be improved in patients with coronary artery disease (CAD)?
Cardiovascular (CV) risk factor control is best achieved as part of coordinated, multidisciplinary programme that should include:1,3,21
- Patient education
- Smoking cessation advice and support
- Lifestyle changes promoting lipid, blood pressure and diabetes control (e.g. regular physical exercise, adopting a healthy diet, following a weight-management programme)
- Use of medical therapies for lipid, blood pressure and diabetes control (e.g. statins, antihypertensives, insulin/glycaemic control medication)
- Psychosocial management, such as social support and treatment of underlying conditions such as depression and anxiety
The COMPASS study has shown that, in patients with CAD, vascular protection with dual pathway inhibition can provide additional protection against myocardial infarction, stroke and CV death compared with an antiplatelet alone.9 Although addition of a second antithrombotic was associated with an increased risk of major bleeding, there were no significant increases in the risks of intracranial haemorrhage and fatal bleeding.9
The COMPASS regimen is indicated for the prevention of atherothrombotic events in adult patients with CAD or peripheral artery disease at high risk of ischaemic events.22
The 2019 ESC guidelines recommend that the COMPASS regimen should be considered for the treatment of patients with chronic coronary syndromes at high risk of ischaemic events and without high risk of bleeding.1
The COMPASS trial explained.
Video explaining the results of the COMPASS trial
PP-XAR-ALL-0516-2
How can outcomes be improved in patients with peripheral artery disease (PAD)?
The overarching aims of the treatment of symptomatic PAD are to reduce symptoms and to improve prognosis.5,6 The management of symptomatic PAD includes lifestyle modifications, medical therapies and, in some instances, revascularization to:
- Improve limb symptoms
- Limit further progression of atherosclerosis through the control of cardiovascular (CV) risk factors
- Prevent potentially fatal ischaemic events
- Minimize tissue loss/need for amputation
A multidisciplinary approach is warranted to establish an effective management strategy for patients with symptomatic PAD.6,11
Antithrombotic therapy is crucial for the protection against thrombotic events in patients with symptomatic PAD, but clinical trials investigating intensified antiplatelet regimens have been largely unsuccessful in patients with symptomatic PAD.23-25
Recently, two large randomized controlled trials have demonstrated the benefits of dual pathway inhibition in patients with PAD. The COMPASS trial included patients with mostly chronic PAD, and the VOYAGER PAD trial included patients with symptomatic PAD undergoing peripheral revascularization procedures.10,17
Patients with PAD in the COMPASS study were found to benefit from vascular protection with dual pathway inhibition compared with an antiplatelet alone.9,10 Not only did this regimen reduce the risk of myocardial infarction, stroke and CV death by 28% versus an antiplatelet alone, it also reduced the risk of major adverse limb events by 46%.9,10 Although the addition of an additional antithrombotic was associated with an increased risk of major bleeding, there were no significant increases in the risks of intracranial haemorrhage or fatal bleeding.9,10 The success of this study led to indication of the COMPASS regimen for the prevention of atherothrombotic events in adult patients with symptomatic PAD at high risk of ischaemic events.22
The benefit of dual pathway inhibition for patients with PAD that were first seen in the COMPASS trial was also investigated in the VOYAGER PAD trial which assessed the efficacy and safety of dual pathway inhibition versus single antiplatelet therapy in patients with symptomatic PAD undergoing lower extremity revascularization.17 The results of the study showed that dual pathway inhibition reduced the risk of composite outcome (acute limb ischaemia, major amputation of vascular aetiology, myocardial infarction, ischaemic stroke or cardiovascular death) by 15% compared with antiplatelet therapy.17 Additionally, TIMI major bleeding did not significantly increase with dual pathway inhibition compared with antiplatelet therapy.17
How do co-morbidities affect the management of atherothrombosis with the COMPASS regimen?
The COMPASS regimen is indicated for the prevention of atherothrombotic events in adult patients with coronary artery disease and symptomatic peripheral artery disease at high risk of ischaemic events.22
In the COMPASS trial, a high baseline risk of events means that patients with heart failure, diabetes and renal impairment (estimated glomerular filtration rate 15–60 ml/min) were among those who derived the largest benefit from vascular protection with dual pathway inhibition compared with an antiplatelet alone.20 (Please see the Which patients can benefit most from vascular protection? section)
References
- Knuuti J, Wijns W, Saraste A et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. Return to content
- Cosentino F, Grant PJ, Aboyans V et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323. Return to content
- Fihn SD, Gardin JM, Abrams J et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:e354–e471. Return to content
- Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention 2015;10:1024–1094. Return to content
- Gerhard-Herman MD, Gornik HL, Barrett C et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2017;69:e726–e779. Return to content
- Aboyans V, Ricco JB, Bartelink MEL et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Eur Heart J 2018;39:763–816. Return to content
- Frank U, Nikol S, Belch J et al. ESVM guideline on peripheral arterial disease. Vasa 2019;48:1–79. Return to content
- Conte MS, Bradbury AW, Kolh P et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 2019;69:3S–125S.e140. Return to content
- Eikelboom JW, Connolly SJ, Bosch J et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–1330. Return to content
- Anand SS, Bosch J, Eikelboom JW et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:219–229. Return to content
- Conte MS, Pomposelli FB, Clair DG et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg 2015;61:2s–41s. Return to content
- Murphy TP, Reynolds MR, Cohen DJ et al. Correlation of patient-reported symptom outcomes and treadmill test outcomes after treatment for aortoiliac claudication. J Vasc Interv Radiol 2013;24:1427–1435. Return to content
- Bodewes TCF, Darling JD, Deery SE et al. Patient selection and perioperative outcomes of bypass and endovascular intervention as first revascularization strategy for infrainguinal arterial disease. J Vasc Surg 2018;67:206–216 e202. Return to content
- Baumgartner I, Norgren L, Fowkes FGR et al. Cardiovascular outcomes after lower extremity endovascular or surgical revascularization: the EUCLID trial. J Am Coll Cardiol 2018;72:1563–1572. Return to content
- Hess CN, Wang TY, Weleski Fu J et al. Long-term outcomes and associations with major adverse limb events after peripheral artery revascularization. J Am Coll Cardiol 2020;75:498–508. Return to content
- Conte MS, Bradbury AW, Kolh P et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019;58:S1–S109 e133. Return to content
- Bonaca MP, Bauersachs RM, Anand SS et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med 2020;382:1994–2004. Return to content
- Esmon CT. Targeting Factor Xa and thrombin: impact on coagulation and beyond. Thromb Haemost 2014;111:625-633. Return to content
- Ramacciotti E, Weitz JI. Rivaroxaban plus aspirin for cardiovascular protection: Rationale for the vascular dose and dual pathway inhibition. Thromb Res 2019;184:44-49. Return to content
- Anand SS, Eikelboom JW, Dyal L et al. Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS trial. J Am Coll Cardiol 2019;73:3271–3280. Return to content
- Cortés-Beringola A, Fitzsimons D, Pelliccia A et al. Planning secondary prevention: room for improvement. Eur J Prev Cardiol 2017;24:22–28. Return to content
- Bayer AG. Xarelto® (rivaroxaban) Summary of Product Characteristics. 2020. Available at: https://www.ema.europa.eu/documents/product-information/xarelto-epar-product-information_en.pdf [accessed 20 November 2020]. Bayer AG. Xarelto® (rivaroxaban) Summary of Product Characteristics. 2020. Available at: https://www.ema.europa.eu/documents/product-information/xarelto-epar-product-information_en.pdf [accessed 20 November 2020]. Return to content
- Cacoub PP, Bhatt DL, Steg PG et al. Patients with peripheral arterial disease in the CHARISMA trial. Eur Heart J 2009;30:192–201. Return to content
- Belch JJ, Dormandy J, CASPAR Writing Committee. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg 2010;52:825–833e822. Return to content
- Hiatt WR, Fowkes FG, Heizer G et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med 2017;376:32–40. Return to content


